Alluring ... Rare ... Cherished
CHOOSING THE PERFECT DIAMOND - 4Cs of DIAMOND QUALITY
Buying your diamond at a memorable and significant time in your life, such as an engagement or wedding, can be mystifying and overwhelming. This Diamond Education section written by Monroe Yorke Diamonds is the key to finding the perfect diamond or diamond jewellery. We have designed this Education section to be helpful, informative and practical. We hope you will find it useful in your search for that perfect diamond for your loved one.
You can be assured that every diamond from Monroe Yorke Diamonds has undergone laboratory grading and evaluation. We will explain the information on Diamond Grading Certificates and how diamonds are graded and what this means to you as a buyer.
In this Diamond Education section, you will find that your questions about diamonds and their characteristics will be answered. We demystify the "4Cs" which forms the cornerstone of the way that diamonds are graded. Consider us your guide on your exciting journey to finding the perfect diamond from Monroe Yorke Diamonds.
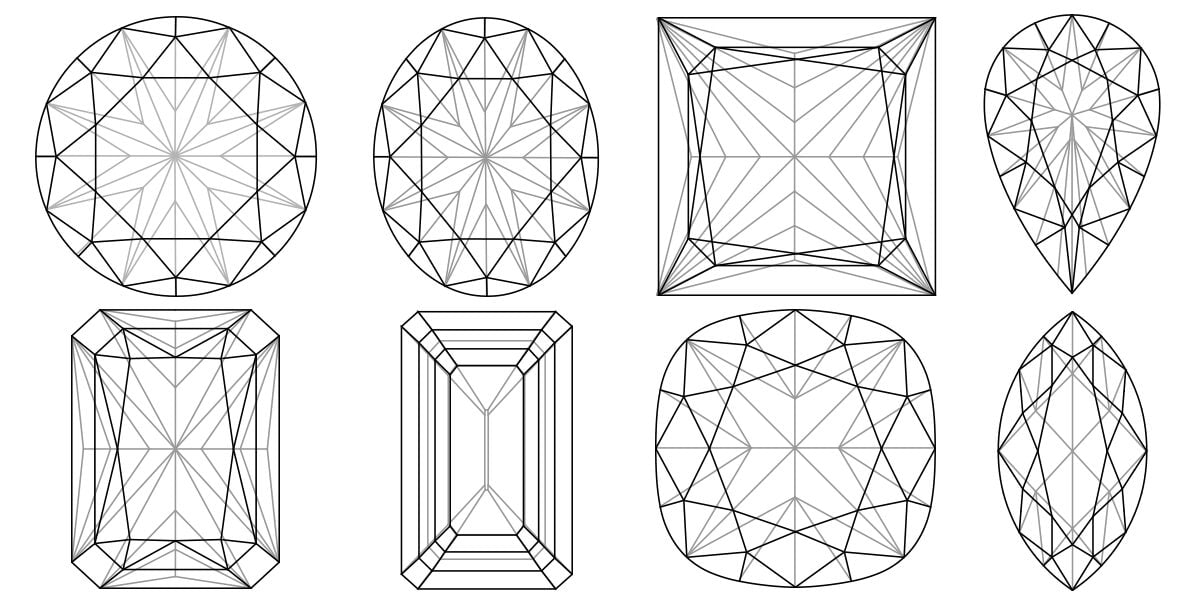
The Colour, Clarity, Cut and Carat Weight of a diamond are collectively termed the 4Cs. These are the factors that, when combined together, define a diamond’s quality and ultimately determine its value. The Gemological Institute of America (GIA) created these 4Cs of diamond quality, which has become the universal method for assessing the quality of any diamond. The creation of the 4Cs means that diamond quality can be communicated in a universal language so that as a buyer you know exactly what you are buying.

Amazing Brilliance, Fire and Scintillation
Characteristics of the Monroe Yorke Diamond
Excellent Cut
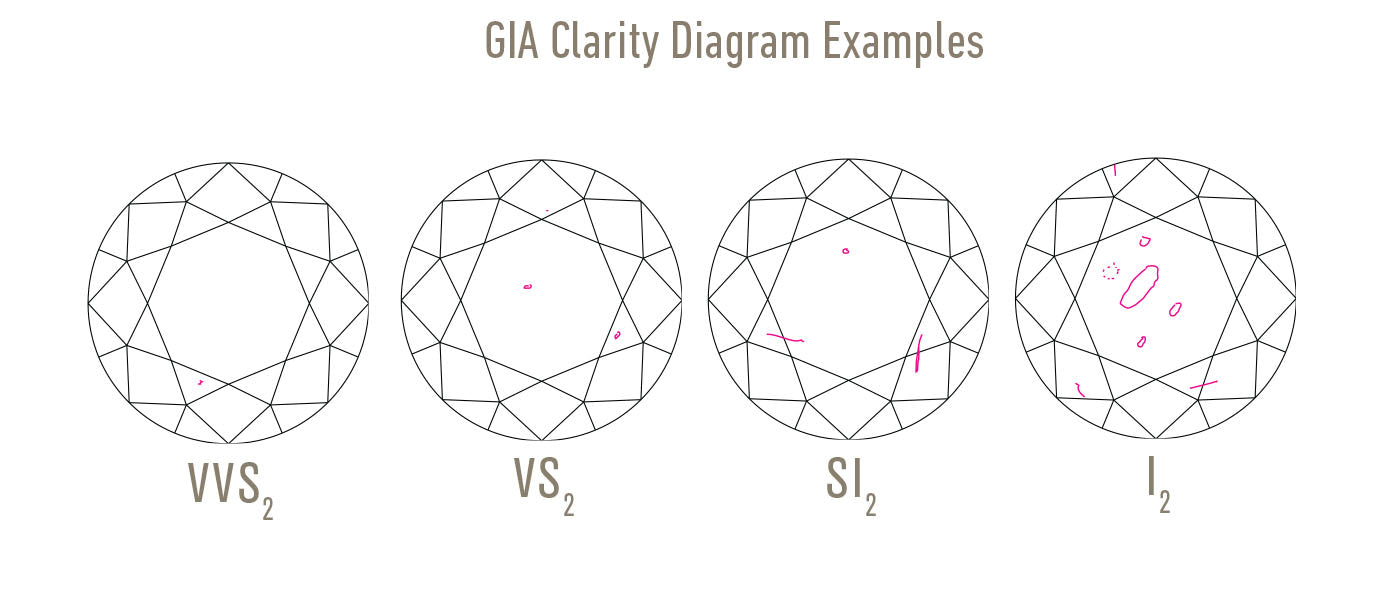
Flawless to SI2 Clarity (eye clean where inclusions only visible at x10 magnification)
Colourless Diamonds - Colours D - J
Independently certified by GIA and laser inscribed by GIA
Independently authenticated and valued by member of National Council of Jewellery Valuers in Australia